Physical Review, Series 1, 16:300(1903).
Recent experiments on the nature of excited radioactivity have shown that by various mechanical and chemical means the radioactivity may be removed from the surface on which it is found, but no proof has been given that it can be destroyed by such treatment. On the contrary, when an excited wire loses its activity by friction, the substance with which it is rubbed becomes radioactive to a degree corresponding to the los from the wire. When attacked by acids, the radioactivity lost from the wire is found in the solvent.
Professor Rutherford has found that a platinum wire excited by thorium emanations can be made red hot without appreciably changing it activity, but that the activity is almost entirely lost after heating the wire to incandescence. This sudden disappearance of the radioactivity at white heat seemed to furnish evidence of it destruction, and with the aim of either establishing or refuting such a view the following experiments were undertaken.
If the excited radioactivity is not destroyed by intense heat, it wa argued that by heating the wire inside a closed vessel so as to prevent all escape, the active particles, although removed from the wire, would still remain and might be discovered in the surrounding air or on the walls of the vessel. For this purpose a hollow brass cylinder about ten inches in length and three inches in diameter was fitted with ebonite ends; in each end were two openings, one in the center through which to pass the wire, and another through which to send a blast of air when desired. The wires used were six inches in length and about one twentieth of an inch in diameter. The cylinder was used in the usual manner, as a testing vessel for measuring the amount of radioactivity within it. Its outside was joined to one terminal of a storage battery of 100 volts, and the central wire to one pair of quadrants of an electrometer of which the other pair was connected to earth. Another testing vessel was used for comparing the amounts of activity produced by the wire before and after heating.
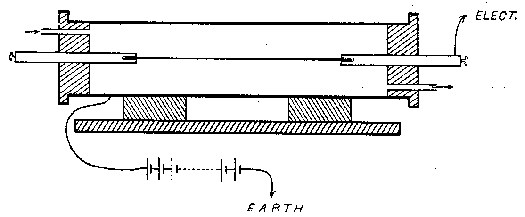
The wire was excited by making it the cathode of a P.D. of 100 volts and placing it for several hours inside a closed metal box containing 50 grams of thoria. When placed in position in the cylinder, an ionization current of the order of 10-11 amperes was produced. A current of 20 amperes was then passed through the wire, heating it to incandescence, after which the ionization current was again measured. Instead of showing a great loss, as might have been expected, the current was nearly as large as before; upon replacing the platinum wire by an inactive copper wire but little further change was observed. The platinum wire was then put in the second testing vessel and found to possess only about 3 per cent of its original activity. Clearly then the active particles had been removed from the wire and were still inside the cylinder. The air was then blown out but no decrease in current wa thereby obtained, showing that the active particles had become attached to the inside surface of the cylinder. The rate of decay of the radioactivity within the cylinder was observed and found to be such that it was reduced to half value in eleven hours, the characteristic of excited radioactivity from thorium.
From these results we conclude that intense heat does not destroy excited radioactivity, and that under conditions as here described the active particles are transferred at a definite temperature without other change from the platinum wire to the inside surface of the cylinder.
The tests were repeated with wires excited by radium emanations with similar results, although on account of the rapid decay of the excited radium radioactivity during the first fifteen minutes, the tests had to be confined to the period between 15 and 35 minutes after the removal of the wire, since the rate of decay is known to be a minimum during thi interval.
Some of the results of several sets of these observations are given in tabular form. (See Table I, 1-4) It can not be assumed that, if all of the active particles were transferred from the wire to the cylinder, the ionization current would necessarily be unchanged, since in the latter case the radiations are not all radial as in the former. It would be difficult to determine experimentally how great a change this produces, but it i doubtless large enough to account for the slight numerical difference between the current before and after the wire is heated, when we also consider that some of the active particles may have escaped through the ends of the cylinder with the sudden outrush of heated air.
In order to get from the wire to the cylinder the active particles must have been in the air at some time, and since none could be blown out later, all must have been transferred during the heating. This was shown experimentally to be the case by the fact that the particles could be blown out of the cylinder while the wire was being heated, although at no other time. A strong blast of dust-free air was directed along the wire all the time it was being heated. Although this so cooled the wire that fewer particles were driven from it than in the preceding tests, the activity within the cylinder was reduced to less than one third it original value, showing that over two thirds of the particles had been driven out of the cylinder. It was found possible to blow the particle from one cylinder into another.
An attempt was made to observe the effect produced by blowing and heating in the presence of a strong electric field with the object of ascertaining the nature of the charges carried by the particles, but no definite conclusions were attained.
Two views may be taken as to the cause of the removal of the active particles from excited platinum at white heat:
It is known that platinum when white hot undergoes a surface disintegration in air, and the remarkable disappearance of the radioactivity at about this temperature is in harmony with the view that the active particles were carried off with the outside disintegrated film of the wire. If this were the true explanation, wires of other material, or even platinum wires when surrounded by other gases, should not show the same effects.
German-silver and platinum-iridium (10 per cent iridium) wires were used instead of platinum, but although the wires lost less of their activity in these cases, the behavior of the liberated particles was the same a that of those expelled from platinum. When the wire was heated in carbon dioxide instead of in air, it lost 97 per cent of its radioactivity, and nearly as much when heated in hydrogen. (See Table I, 5-8; Table II, 7)
These results seem to completely refute the theory that a disintegration of platinum is necessary to cause the transfer of the excited radioactivity from the wires to the walls of the surrounding vessel and to point to the conclusion that, at the temperature of white heat for platinum, a volatilization of the active material itself takes place.
During the course of the above observations an interesting effect wa noticed which has not been mentioned. When measurements of the ionization current were made immediately after heating, the current wa always much less than the value which it afterward attained, sometime being only a third that amount. It would then steadily increase for twenty or twenty five minutes when the maximum was usually reached. It can easily be supposed that part of this effect is due to the cooling of the air since the ionization current varies inversely as the absolute temperature, but neither could so large a change be accounted for on this ground alone, nor would the current increase for so long a period. The phenomenon is completely explained by assuming that the air in the cylinder after heating is filled with particles of disintegrated platinum and other matter, which behaving as dust particles increase the rate of recombination and decrease the current for a given voltage. That this assumption is correct was shown by blowing a current of dustles air through the cylinder immediately after heating. The values which otherwise required twenty minutes to be reached, were attained immediately.
Summary of Results:
I desire to express my gratitude to Professor Rutherford through whose kindness the privileges of this laboratory have been extended to me, and under whose direction these experiments have been performed.
MacDonald Physics Building, McGill University, Montreal, January, 1903.
| Exp. No. | Wire Used | Excited Radioactivity Due to | Activity from Wire Before Heating | From Cylinder and Wire After Heating | From Cylinder Alone After Heating | From Wire Alone After Heating | Difference |
|---|---|---|---|---|---|---|---|
| 1 | Platinum | Thorium | 100 | 90 | 87 | 2 | 10 |
| 2 | " | " | 100 | 92 | 90 | 2 | 8 |
| 3 | " | " | 100 | 94 | - | - | 6 |
| 4 | " | Radium | 100 | 92 | 88 | 2 | 8 |
| 5 | German Silver | " | 100 | 98 | 33 | 66 | 2 |
| 6 | Platinum iridium | Thorium | 100 | 97 | 81 | 15 | 3 |
| 7 | Platinum in CO2 | " | 100 | 98 | 95 | 3 | 2 |
| 8 | Platinum in H | " | 100 | 95 | 89 | 6 | 5 |
| Exp. No. | Wire Used | Excited Radioactivity Due to | Activity from Wire Before Heating | From Cylinder and Wire After Heating | From Cylinder Alone After Heating | From Wire Alone After Heating | Difference |
|---|---|---|---|---|---|---|---|
| 1 | Platinum | Thorium | 100 | 90 | 87 | 2 | 10 |
| 2 | " | " | 100 | 92 | 90 | 2 | 8 |
| 3 | " | " | 100 | 94 | - | - | 6 |
| 4 | " | Radium | 100 | 92 | 88 | 2 | 8 |
| 5 | German Silver | " | 100 | 98 | 33 | 66 | 2 |
| 6 | Platinum iridium | Thorium | 100 | 97 | 81 | 15 | 3 |
| 7 | Platinum in CO2 | " | 100 | 98 | 95 | 3 | 2 |
| 8 | Platinum in H | " | 100 | 95 | 89 | 6 | 5 |
| Exp. No. | Wire Used | Excited Radioactivity Due to | Activity from Wire Before Heating | From Cylinder and Wire After Heating | From Cylinder Alone After Heating | From Wire Along After Heating | Amount Escaped |
|---|---|---|---|---|---|---|---|
| 1 | Platinum | Thorium | 100 | 36 | 20 | 18 | 64 |
| 2 | " | " | 100 | 36 | 10 | 13 | 64 |
| 3 | " | " | 100 | 38 | 20 | 18 | 62 |
| 4 | " | " | 100 | 22 | 16 | 6 | 78 |
| 5 | " | Radium | 100 | 25 | - | - | 75 |
| 6 | " | " | 100 | 23 | 15 | 8 | 77 |
| 7 | Platinum iridium | " | 100 | 45 | 31 | 14 | 55 |